Using ongoing newborn intervention trials to obtain additional data critical to maternal, fetal and newborn health in a harmonized way in Sylhet District, Bangladesh: the AMANHI (Alliance for Maternal and Newborn Health Improvement) cohort study.
Study Period: August 2014-June 2026
Sample Size: 3000Donor Name:Bill and Melinda Gates Foundation (BMGF) through World Health Organization (WHO).
Project Description:
Background:The umbrella study, which is being conducted by the Alliance for Maternal and Newborn Health Improvement (AMANHI), aims to better understand the epidemiology of maternal morbidity and mortality, stillbirths, and neonatal deaths, the relationships between maternal morbidity and care and maternal, fetal, and neonatal outcomes, the biological underpinnings of these relationships, and the evaluation of less complex methods for accurate gestational age determination in several countries including Bangladesh and coordinated by WHO.
Objective: i) Identify causes of maternal, and neonatal deaths and stillbirths using standardized verbal autopsy method;
ii) Measure morbidities during pregnancy, childbirths and postpartum period (e.g., pre-eclampsia, eclampsia) and identify their association with adverse pregnancy outcomes (e.g. stillbirths, preterm births and intrauterine growth retardation);
iii) Identify simpler tools to detect preterm births by community health workers; and
iv) Create a biorepository of maternal, neonatal and paternal biological samples to detect biological and genetic markers of adverse pregnancy outcomes.
In 2016, the study received additional support to include child growth and development outcomes (All Children Thrive; ACT). The ACT study involves continued enrollment and follow-up of pregnant women, collection of some additional specimens to be added to the biorepository, follow-up of the babies born to the mothers and longitudinally assess their growth and neurodevelopment during first 3 years of life. The main aim of ACT is to measure the trajectory of growth and development in a well-defined cohort of preterm birth, growth restricted, and normal newborns in order to find the underlying biological mechanisms and/or predictors of differential growth and performance on test of cognitive, motor and language development.
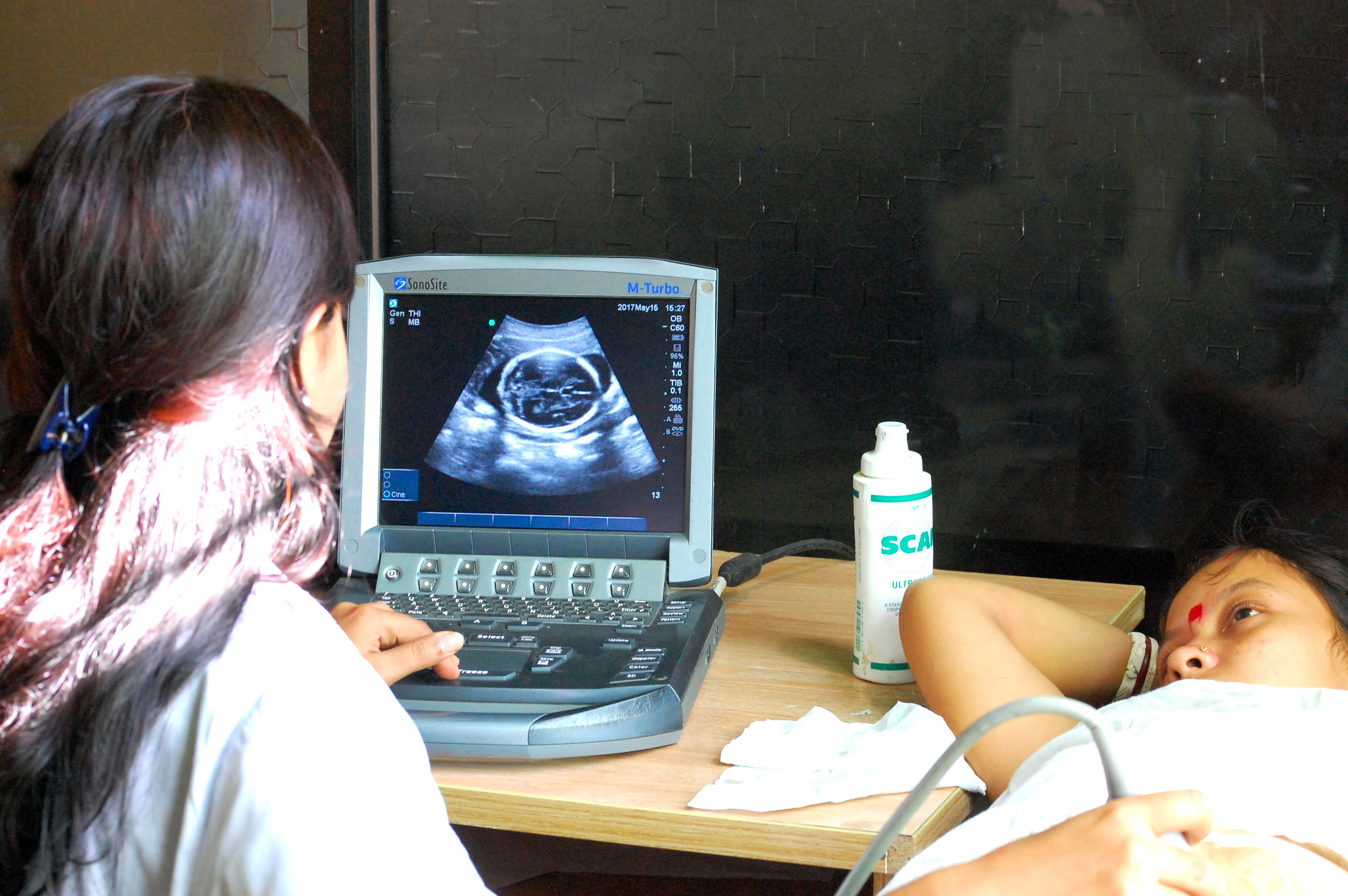
Method:The AMANHI Biorepository study is being implemented in Bangladesh, Pakistan and Tanzania to initially test hypothesized biological markers as predictors of important maternal, fetal, and child health outcomes (e.g., pre-eclampsia, eclampsia, stillbirths, preterm births and intrauterine growth retardation), and to use this opportunity to establish a repository of biological samples for testing as new hypotheses, methods and technologies become available.
Between 21 August 2014 and 30 August 2017, we have enrolled 3,000 women in early pregnancies (8-19 weeks of gestation) in Sylhet, Bangladesh, collected biospecimens from the enrolled women, their husbands, and babies and stored the samples at -800C in the Biobanks located at Sylhet and Dhaka. The Biobank contains various specimens including maternal and umbilical cord blood, plasma, serum, urine, placental tissue, saliva, and stool that are amenable to proteomic, transcriptomic, metabolomic, genomic and microbiomic analyses. All these samples will be linked to numerous epidemiological and phenotypic data with unique study identification numbers.
We are now collaborating with other AMANHI sites, consortiums and several US-based technology partners to identify markers of preterm birth using the bio-specimens that we have collected. Our collaborative work with the University of Iowa is to test existing models of gestational age prediction using newborn metabolic profiles and build new models using bio-banked cord blood specimens. We are collaborating with the Stanford University to examine whether the blood and urine samples can be used for state-of-the-art biologic analytic inquiries to identify proteomic, transcriptomic and metabolomic markers of preterm birth. The collaborative work with Cincinnati Children’s Hospital Medical Center is to determine the genetic variants that influence preterm birth risk in women. Our collaboration with a US company, Sera Prognostics, is aimed at identifying proteomic markers of preterm birth and ultimately to develop a point of care diagnostic test for the accurate and early prediction of preterm birth in early pregnancy.
The AMANHI method papers have been published in 2016 and 2017 (AMANHI Mortality Cohort Study Group, J Glob Health. 2016; AMANHI Maternal Morbidity Study Group, J Glob Health. 2016; AMANHI Gestational Age Study Group, J Glob Health. 2017; AMANHI Bio-banking Study Group, J Glob Health. 2017). The paper on population-based rates, timing, and causes of maternal deaths, stillbirths, and neonatal deaths in south Asia and sub-Saharan Africa has been published in 2018 (AMANHI Mortality Study Group, Lancet Glob Health. 2018). The paper on direct maternal morbidity and the risk of pregnancy-related deaths, stillbirths, and neonatal deaths has recently been published in 2021 (AMANHI Maternal Morbidity Study Group, PLOS Medicine. 2021).
